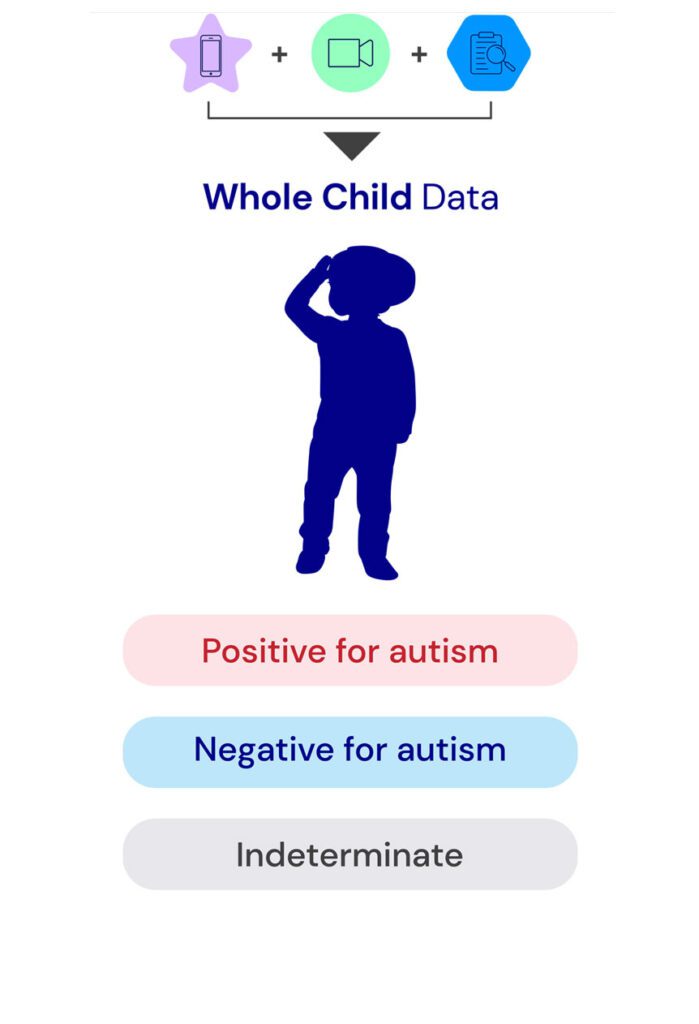
We know that when there is early concern, every moment counts.
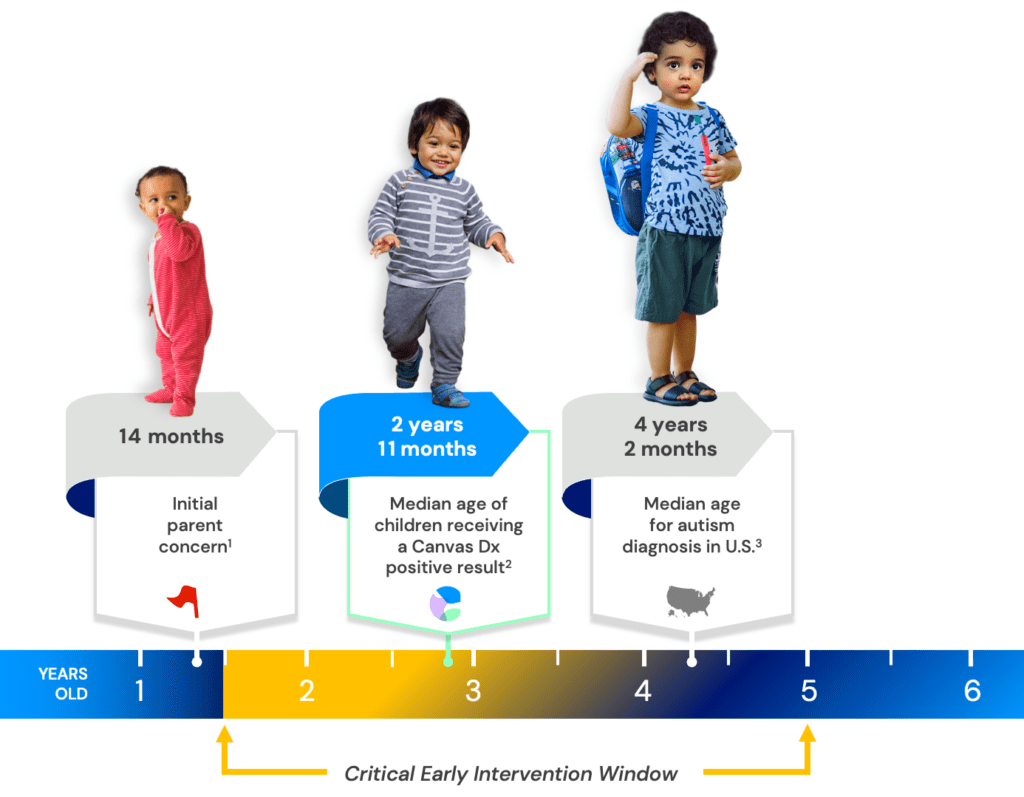
But too many children are diagnosed long after they have outgrown the critical early neurodevelopmental window when interventions have the greatest life-changing impact.

Our breakthrough diagnostic technology allows you to more definitively understand your kids’ developmental picture, so you can take earlier action to better support them.
FOR PRESCRIPTION USE ONLY
Introducing Canvas Dx, the first and only FDA authorized diagnostic that equips more clinicians to rapidly and accurately diagnose or rule out autism in children ages 1.5 to 6 years, better plan for next steps, and unlock services.
 High Accuracy
High Accuracy Clinically proven, rigorously trained & tested, and peer-reviewed, Canvas Dx generates a highly accurate diagnostic prediction for confident clinical decision making.
 Actionable Insights for Every Child
Actionable Insights for Every Child A detailed report identifies developmental strengths and concerns, so you can make informed decisions for all children – with and without autism, and those needing further assessment.
 Fully Remote Experience for Families
Fully Remote Experience for Families No in-person visits required. Reach your patient families where they are. Designed for both primary and secondary care settings alike.
 Easy to Use
Easy to Use Families download a simple app on their smartphone. You are equipped with an intuitive, resourceful web portal. No special training nor certification required.
 Expert Support
Expert Support A dedicated care team supports you and your patient families, from activation to documentation to unlock services with reimbursement from healthcare insurers.
 Fast, Automated Documentation
Fast, Automated Documentation Canvas Dx automatically maps concerns to DSM-5 compatible criteria for autism diagnosis. Save time with built in diagnostic report creation for fast documentation required for services and reimbursement.
 Equitable Diagnoses
Equitable Diagnoses Trained on diverse data from thousands of children at risk for and with developmental delays including autism, Canvas Dx supports objective, equitable evaluation of children of different racial/ethnic backgrounds, across genders, socio-economic status, and geography.
Canvas Dx is now reimbursed by Highmark commercial plans
I want to use Canvas Dx at my patients’ next well check visits, before autism goes unnoticed:
Canvas Dx is simple to use
- The caregiver uses a simple app to answer questions and record and upload two home videos – all from the comfort of their own home.
- The clinician completes a questionnaire about the child's development and behavior in a web portal.
- Canvas Dx does the rest. Our AI combines all this data to generate a highly accurate prediction of autism and additional information of the child's developmental and behavioral strengths and concerns.
- The clinician can immediately review the Canvas Dx result and child development report in the web portal. Diagnostic report-building features saves time to complete required documentation to support access to services and reimbursement.
See how Canvas Dx works
Know more sooner, so you can act earlier.
Now, your first action when there is early developmental concern can be diagnosis. Canvas Dx is the crucial first step to understand and meet your patients’ needs, so kids can flourish and live their most fulfilling lives.